Describe Three Ways to Separate a Mixture
Sedimentation is the process of separating the insoluble solid from the liquid by. Mixtures can be separated using various separation methods such filtrationseparating funnelsublimationsimple distillation and paper chromatography.

Separating Mixtures Science Educational School Posters Chemistry Classroom Gcse Science Teaching Chemistry
Others are made of parts too small to separate by hand.
. Name two products that come from the lighter parts of petroleum. Ways to separate mixtures. Chromatography involves solvent separation on a solid medium.
Up to 24 cash back 2. If you have one of these conditions you might also experience. Describe one way to separate each of the following mixtures.
There are also chemical methods which are used by rearranging the particles so a certain substance no longer exists chemical reaction. After its dissolved you will then filter it. Sorting floating settling using a magnet using sieves and filters dissolving.
To separate the components a mixture of salt and sand is heated above 801C yet below 1710C. Mixtures are made up of both solids and liquids. Mixtures that contain only solids must be separated through sublimation extraction magnetic separation or chromatography.
One way to separate a soluble solid from its solution is to make crystals. The centrifugation method is used to strain the tiny suspended particles which could not be captured by the filtration method. Another common separation process is called distillation.
Method B any three from. Crystalization 7ch SlideShare uses cookies to improve functionality and performance and to provide you with relevant advertising. A filter - air will move through the filter but the dust will be trapped.
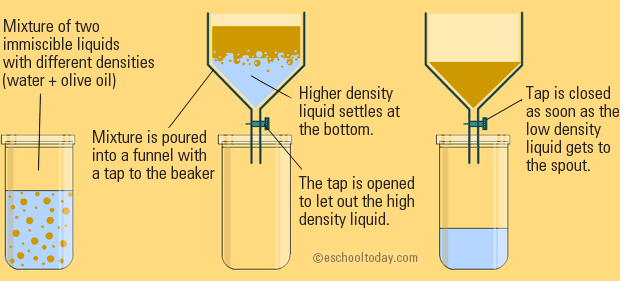
Distillation also make use of heat to separate the particles. This collects pure samples of each solid. Extraction separation of liquids by density and solubility.
Distillation uses boiling to separate mixtures of liquid solutions. Filtration 2mechanical separation 3. D dissolving - sugar will dissolve in the water leaving the sand behind.
C settling - the soil will settle to the bottom of the container of water. Another method to separate components of a mixture is based on melting point. Then leave it to dry and evaporate into a solid.
Evaporation removes a liquid from a solution to leave a solid material. There are different ways that you can separate mechanical mixtures. Make the Dry Mixture Label one cup Dry Mixture Group Add one 5-ml spoon each of.
The evaporation method is used to separate the solid particle from the liquid by heating. Hand sorting is a suitable separation method for a mixture that contains a relatively small number of large items. It can be used to obtain a product that.
B magnet - magnet will pick up the paper clips but not the erasers. Filtration separates solids of different sizes. Add xylene to mixture 1 stir and filter 1 NaNO3 insoluble in xylene so left as residue on filter paper 1 wash NaNO3 with xylene and leave to dry 1 evaporate xylene from filtrate of S solution 1 by warming on water bath electrically heated no naked.
One way to separate a mixture of sulphite powder and sodium nitrate is to mix them with water until it dissolves. The two main ways to avoid water pollution are to identify and limit water pollution sources and to treat polluted. Distillation takes advantage of differences in boiling points.
This technique is used to separate an insoluble solid from a liquid. Ways to separate Mixtures and Solutions Evaporation Filtration Separating Funnel Manual Separation Paper Chromatography Magenetism MANUAL SEPARATION A separation process that removes isolates or separates a substance from a sample by methods such as extraction or distillation. Salt becomes molten at a lower temperature than sand.
Mixtures can be physically separated by using methods that use differences in physical properties to separate the components of the mixture such as evaporation distillation filtration and chromatography. Settling is used in primary sewage treatment to separate solid wastes from liquid waste. The melting point of salt is 1474F 801C while that of sand is 3110F 1710C.
Mixtures that contain only liquids must be separated through fractional distillation or gravity separation. There are three main steps in. Separating Mixtures Overview Common Methods Expii Bipolar disorder affects each person in different ways.
The methods stated above are all physical methods. The particles that are removed from the water by the filter are called the residue. When we want to separate a mixture we can use the differences in the physical properties of the components of the mixture to separate the components from each other.
The water that has been run through the filter is called the filtrate. Passing a mixture through a screen or filter will allow the small particles to pass and be separated from the larger particles that get trapped. 1 separation by size 2 transfer to a solid support and 3 marking target protein using a proper primary and secondary antibody to visualize.
There are different ways to separate a mixture including chromatography filtration distillation sublimation solvent extraction. A wheat grains with large sticks b wheat grains with metal pieces 3. Up to 24 cash back SEPARATINGMECHANICALMIXTURES.
Mixtures can be separated using a variety of techniques. Gravel Powder Salt Stir the dry mixture Plan how you will separate the mixture Write a detailed plan in your Science Notebook - include materials needed and. Some mechanical mixtures can be separated by hand.
Different ways in order for us to separate mixtures.

1 4 Laboratory Techniques For Separation Of Mixtures Chem 1114 Introduction To Chemistry


Comments
Post a Comment